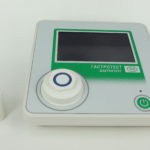
The “GastroTest” device
PRICE:
PRICE:
0 руб
The “GastroTest” device is intended for determination of presence of bacterium Helicobacter pylori in the human organism by controlling the change in ammonia content in the exhaled air. The principle of action of the device: the investigation determines the activity of enzyme urease produced by the bacteria Helicobacter pylori in the patient’s stomach.
The infection index is the test result. The infection index is an indicator of exceeding of ammonia content in the human exhaled air measured after intake of carbamide solution relative to baseline ammonia content in the human exhaled air. The infection index is expressed in percent.
- Registration certificate for a medical device РЗН 2021/13993 от 12.04.2021
- Patent №194744
- ISO 13485: 2016 Certificate №71860
- TU 26.60.12-001-16796024-2019
- Description
- Appointment
- Target segments
- Комплектация
- Документация
Description
Main technical characteristics
| The range of determined ammonia concentrations | from 0 to 20 mg/m3 |
| The infection index | 0 ÷ 20 – No urease activity; 21 and above – High urease activity |
| Специфичность | 92% |
| Overall dimensions (L×W×H) | 180×180×92 mm |
| The device weight | 1,0 kg |
| Time of one examination | no more 15 minutes |
| Number of saved results | 300 pcs |
| Mode of operation | 8 hours (15 minutes – work and 10 minutes – break, when powered by AC) |
| Average device life | at least 5 years |
| Results transmission | Bluetooth 4.0 not lower than 4.0 (except IOS); Wi-Fi; Ethernet |
| Instrument power supply | Built-in rechargeable battery; From the mains via the mains adapter |
| Battery charging time | no more than 5 hours |
| Fully charged battery operating time | not less than 3 hours |
In the implementation of the “GastoTest” device, financial support was provided by the Innovation Support Fund.
The “GastroTest” device is intended for determination of presence of bacterium Helicobacter pylori in the human organism by controlling the change in ammonia content in the exhaled air. The principle of action of the device: the investigation determines the activity of enzyme urease produced by the bacteria Helicobacter pylori in the patient’s stomach.
The infection index is the test result. The infection index is an indicator of exceeding of ammonia content in the human exhaled air measured after intake of carbamide solution relative to baseline ammonia content in the human exhaled air. The infection index is expressed in percent.
- Medical centers (hospitals, outpatient clinics, health resorts, testing under “The screening” program).
- Departmental medical units (ministry of defense, ministry of internal affairs, ministry of emergency situations).
- Personal users (pharmacy, dealers, leasing companies, online shops, general practitioners, family therapists).
- Прибор “Гастротест”
- Сменный измерительный модуль
- Сетевой адаптер питания
- Сетевой кабель
- Кабель USB
- Набор на 100 обследований (мундштуки, порции карбамида, пробозаборный капилляр, стаканчики одноразовые, ложки одноразовые, стаканчики мерные).
- Руководство по эксплуатации с паспортом
- Памятка пациенту